
If there are more electrons than protons, the ion has a negative charge and is called an anion.Įlements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). If there are more protons than electrons, an atomic ion has a positive charge and is called a cation. Cesium-135 Cs CID 6335805 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety. Rewrite the equation as 55+N 133 55 + N 133. Fill in the known values where N N represents the number of neutrons. Since cesium cesium 's atomic number is 55 55, Cs Cs has 55 55 protons. Number of electron: 55 Number of Neutrons: 78 Valency: +1 Common isotopes: The only naturally, stable occurring isotope of this element is Cesium-133. There are also at least 39 artificial isotopes created in a. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons-or not.Īn ion of an atom is one in which the number of protons and electrons is not the same. Next, find the atomic number which is located above the element 's symbol. Boiling point: 1,239.8 F (671 C) Number of natural isotopes (atoms of the same element with a different number of neutrons): 1. Caesium is a chemical element with atomic number 55 which means there are 55 protons in its nucleus. Remember, a neutral atom contains the same number of protons and electrons. The upper right side shows the number of electrons in a neutral atom. The element atomic number and name are listed in the upper left. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The electron shells are shown, moving outward from the nucleus.

Here are electron shell atom diagrams for the elements, ordered by increasing atomic number.įor each electron shell atom diagram, the element symbol is listed in the nucleus. 55 electrons (green) bind to the nucleus, with a single, relatively unstable electron in the outer shell (ring). The nucleus consists of 55 protons (red) and 78 neutrons (blue). The reactor fuel is artificially enriched so that it contains 3 235 U. Diagram of the nuclear composition and electron configuration of an atom of caesium-133 (atomic number: 55), the most common isotope of this element. Natural uranium contains 0.7 of the isotope 235 U, the remaining 99.3 being 238 U, which is not fissionable by thermal neutrons.
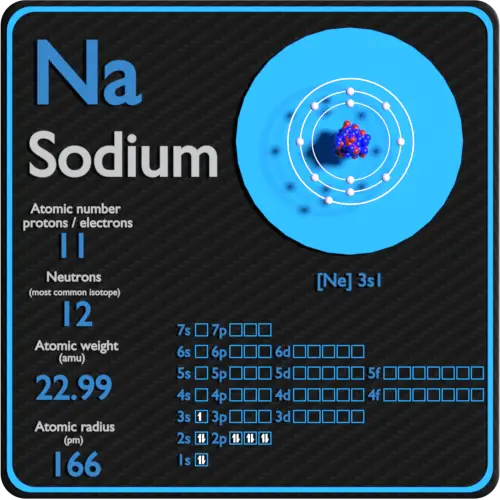
For that, we have electron shell diagrams. Reactors based on the fission of 235 U by thermal neutrons use enriched uranium. The word 'cesium' is derived from caesium (Latin for 'sky blue'). Cesium is an alkali metal that reacts explosively with water and melts just above room temperature. This isotope is stable and thus has no decay products, so instead we show decay chains that lead down to it.Ĭlick any isotope in diagram to see its data.ĭecay chain image generated by Mathematica's GraphPlot and IsotopeData functions from Wolfram Research, Inc.It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. melting point: 28.5 ° C boiling point: 671 ° C density: 1.873 g/cm 3 most common ions: Cs +. Isotope data for cesium-133 in the Periodic Table H Atomic Number: 55 Symbol: Cs Atomic Weight: 132.


 0 kommentar(er)
0 kommentar(er)
